Project
Improved Diagnostics of Respiratory Flows Using a Lattice-Boltzmann Method and Machine Learning Techniques
Numerical methods have the potential to support and improve classical clinical diagnostics. They enable to investigate a patients’ condition in a detail that in vivo diagnostics cannot provide. The continuously increasing computing power of high-performance computing (HPC) systems has led to new and highly efficient numerical methods to simulate more and more complex physical phenomena. In the last few years, this trend is accelerated by the penetration of machine learning (ML) into the realm of computational fluid dynamics (CFD). Such algorithms are capable of providing rapid feedback to the scientist/user in some cases even faster and more accurate than numerical methods. They are well suited to automate processes and to automatically extract important features from large amounts of data, frequently produced by numerical simulations. The success of ML algorithms depends, however, on the quality of the training data, i.e., in the end it is essential to focus on the continuous hand-in-hand advancement of both ML and simulation methods. It is the objective of the research projects CFD and ML approaches for improved diagnostics in rhinology (AM-SIT) and Multi-physics model of the nasal airflow (MMNA) to develop new numerical methods intelligently combining CFD and ML techniques for rhinology, and to bring those to clinical application.
Project Details
Project term
January 1, 2021–December 31, 2021
Affiliations
Forschungszentrum Jülich
Institute
Jülich Supercomputing Centre
Principal Investigator
Methods
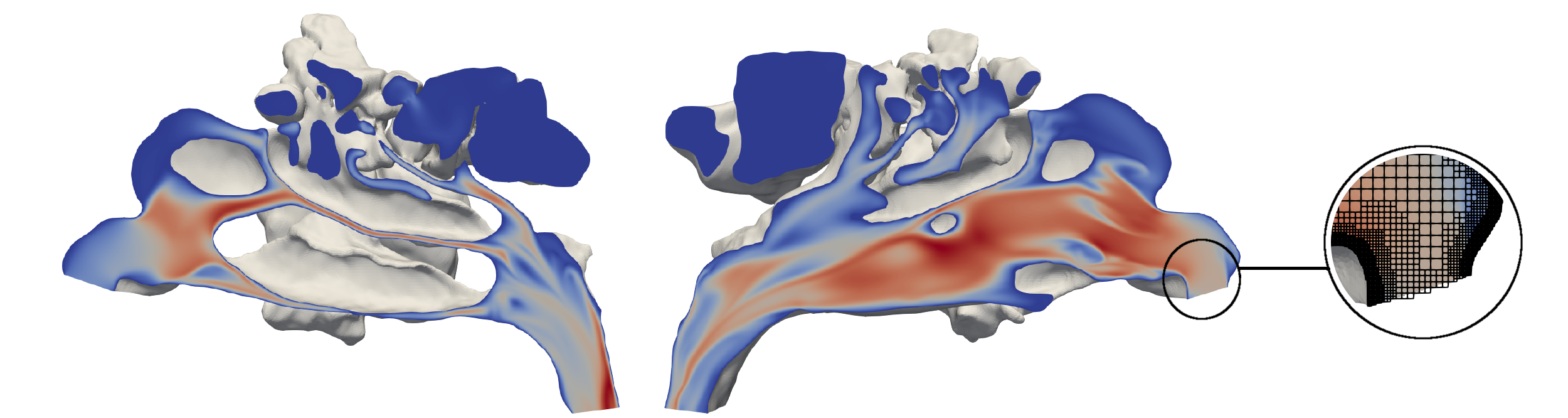
The simulations that have been performed in 2021 are based on a thermal lattice-Boltzmann (TLB) and a level-set (LS) method. They operate on hierarchical unstructured Cartesian meshes. The TLB, as well as the LS methods, are part of the simulation framework multiphysics Aerodynamisches Institut Aachen (m-AIA), jointly developed by the Institute of Aerodynamics and Chair of Fluid Mechanics (AIA), RWTH Aachen University, and the SDL Highly Scalable Fluids & Solids Engineering (SDL FSE), Jülich Supercomputing Centre (JSC).
AM-SIT
CFD simulations have the disadvantage that they usually start from an equilibrium state and need time to reach a fully developed and/or converged state. Therefore, it was studied how flow field approximations generated by Convolutional Neural Networks (CNN) help to overcome this initialization period. The training data (flow simulations for 40 nasal cavities) used for the study was generated on CLAIX using m-AIA. The training itself was done on the GPU partition of JURECA-DC. The large domain size of the highly resolved simulations necessitates to distribute a CNN to multiple GPUs and use patches of the domain as training data. In more detail, an encoder-decoder type fully connected CNN is split to have the encoder branch executed on a single GPU, and the three decoder branches to be distributed across three further GPUs. The decoder branches predict particle probability distributions functions (PPDFs), which are necessary to compute the macroscopic flow variables. The encoder branch takes the Cartesian coordinates of a cell, the distance of a cell to the next boundary, or PPDFs at their boundaries as input. At the moment, the training of the CNN is still ongoing.
MMNA
Virtual surgery using the TLB-LS approach
To test the virtual surgery environment based on the TLB and LS method, a simplified test case is used. A three-dimensional stenotic pipe is chosen to model a nasal cavity with a constriction. During the virtual surgery the stenosis of the pipe was fully removed. That is, the stenotic pipe is simulated for 300,000 iterations to obtain a converged steady state. Based on this solution, the modification of the geometry, which took another 200,000 iterations, is initiated. The simulation outcome before and during the modification is in good agreement with steady state simulations using m-AIA and literature findings.
The virtual surgery in the nasal cavity uses the same simulation methods and wall boundary condition as the stenotic pipe case. At the pharynx a volumetric flow rate of was prescribed. The grid resolution (0.1 mm) is finer than the spatial resolution of the CT data (0.34 mm,0.34 mm,0.75 mm) and resolves all relevant flow features. Again, a steady state simulation for the pre-surgical geometry was performed before the geometry modification was made. During the modification phase, a bone spur was removed, and the deviated septum was straightened. The results show a decreased respiratory resistance after the surgery [2].
Results
Comparison of simulative and RL-based results
The virtual surgery approach proposed in Sec. 2.2.1 was further developed using a reinforcement learning (RL) algorithm to optimize the geometry of a channel with a constriction based on the pressure loss and the heating of the fluid. Since these two parameters have competitive goals, it was expected, that an optimal constriction can be found where the pressure drop is minimized while the heating of the fluid is maximized. The simulations are conducted on 2 nodes of CLAIX. To analyze the behavior of the RL algorithm, a number of 3,400 simulations using m-AIA were conducted, in which the size of the constriction of the channel was continuously increased. From the simulation outcome, a correlation between pressure loss/heating and the size of the constriction was derived. This correlation was defined as the reference solution and compared to the simulation outcome of the RL. The performance of the agent is evaluated in terms of the averaged size of the predicted optimal constriction at each batch. At 69 batches (1,450 simulations using the RL), the agent misses the reference solution by only 0.1%. From here on the error remains below 0.1% for the rest of the training [3]. This study is currently extended to 3D flows.
Additional Project Information
DFG classification: 201 Basic Research in Biology and Medicine
Publications
[1] Rüttgers, M., Waldmann, M., Schröder, W., Lintermann, A.: A machine-learning-based method for automatizing lattice-Boltzmann simulations of respiratory flows, Applied intelligence (2022), doi:10.1007/s10489-021-02808-2
[2] Waldmann, M., Rüttgers, M., Lintermann, A., Schröder W.: Virtual Surgeries of Nasal Cavities Using a Coupled Lattice-Boltzmann–Level-Set Approach, ASME Journal of Medical Diagnostics 5(3) (2022), doi:10.1115/1.4054042
[3] Rüttgers, M., Waldmann, M., Schröder, W., Lintermann, A.: Machine-Learning-Based Control of Perturbed and Heated Channel Flows, High Performance Computing / Jagode, Heike (Editor) ISC High Performance 2021 (2021), doi:10.1007/978-3-030-90539-2_1
[4] Aljawad, H., Rüttgers, M., Lintermann, A., Schröder, W., Lee, K. C.: Effects of the Nasal Cavity Complexity on the Pharyngeal Airway Fluid Mechanics: A Computational Study, Journal of digital imaging 34 (2021), doi:10.1007/s10278-021-00501-x