Project
High Electric Fields in Pyrochlore Materials
With the continuing trend towards miniaturisation of electronic and electrochemical devices, physical effects that can be neglected for µm-scaled devices become important
and even useful at the nanoscale. One such effect is the development of high electric fields when voltages of several volts (typical operating voltages) are applied to nm-thick materials. These fields can lead in principle to increased conductivity in two ways: by increasing ionic mobility, but also by increasing charge carrier concentrations. In this project, we used Gd2Zr2O7, an oxide pyrochlore, as a model system for studying field-dependent defect formation. Its main advantage over other relevant oxide ion conducting materials is the presence of structural vacancies that allow easier defect formation.
The project was divided into two parts: The first part dealt with the description of defect diffusion and formation without an applied electric field. In this way, we were able to obtain a basic description of the system at a given temperature. In the second part, different electric field strengths were applied and the mobility of the oxygen ions and their concentration were followed as a function of the field strength. The final goal was to derive a mathematical expression that describes the field-dependent defect formation as a function of temperature and field strength.
Project Details
Project term
December 1, 2021–November 30, 2022
Affiliations
RWTH Aachen University
Institute
Institute of Physical Chemistry
Principal Investigator
Methods
We have used molecular dynamics (MD) simulations to study oxygen mobility and defect formation by strong electric fields. Due to the high energy required for defect formation (several electron volts), only a small percentage of defects are generated. Therefore, it is necessary to perform the simulations with large supercells containing more than 105 particles. Post-processing involved the identification of defects in the simulation cells, which was carried out using the Wigner–Seitz cell method.
Results
In the first part of the project, we successfully simulated defect formation and diffusion in Gd2Zr2O7 without an applied electric field. The diffusion coefficients and the equilibrium number of defects at each temperature were determined in post-processing and the activation enthalpies of defect migration and formation were calculated. In the second part, we applied high electric fields and derived field-dependent defect mobilities. Using the standard treatment to describe this behaviour, we already observed a positive deviation of our simulation data from the prediction, indicating a higher defect concentration. By comparing the field-dependent defect numbers with the equilibrium numbers derived in the first part, we were finally able to prove that the defect number was indeed increased when high field strengths were applied.
Discussion
In Figure 1, the number of defect pairs formed (aF refers to the type of defect formation reaction, here anti-Frenkel) is plotted as a function of electric field strength (E) at different temperatures (shown with different colours). In addition, the equilibrium number of defects at each temperature is shown with dashed lines. The comparison of the data points with the dashed lines clearly shows that additional pairs of defects are formed at high field strengths. The number of defects was increased by up to an order of magnitude at the highest field strength, independent of temperature.
This means that in principle, by applying high field strengths, the conductivity of oxide ion conducting materials can be increased simultaneously in two ways: by field-enhanced defect formation and migration.
While we successfully demonstrated that the electric field increases the number of defects, the derivation of a quantitative description of the field-dependent defect formation and the subsequent description of the field-dependent mobilities was not achieved within the scope of this project. Future computational time projects will help us collect more data to reliably derive this quantitative relationship. Once this relationship is established, further projects will address the influence of different cations, e.g. La instead of Gd, or cation disorder, e.g. Gd on a Zr site and vice versa, on field-dependent defect formation.
Additional Project Information
DFG classification: 302-03 Chemical Solid State and Surface Research, Theory and Modelling
Software: LAMMPS, OVITO
Cluster: CLAIX
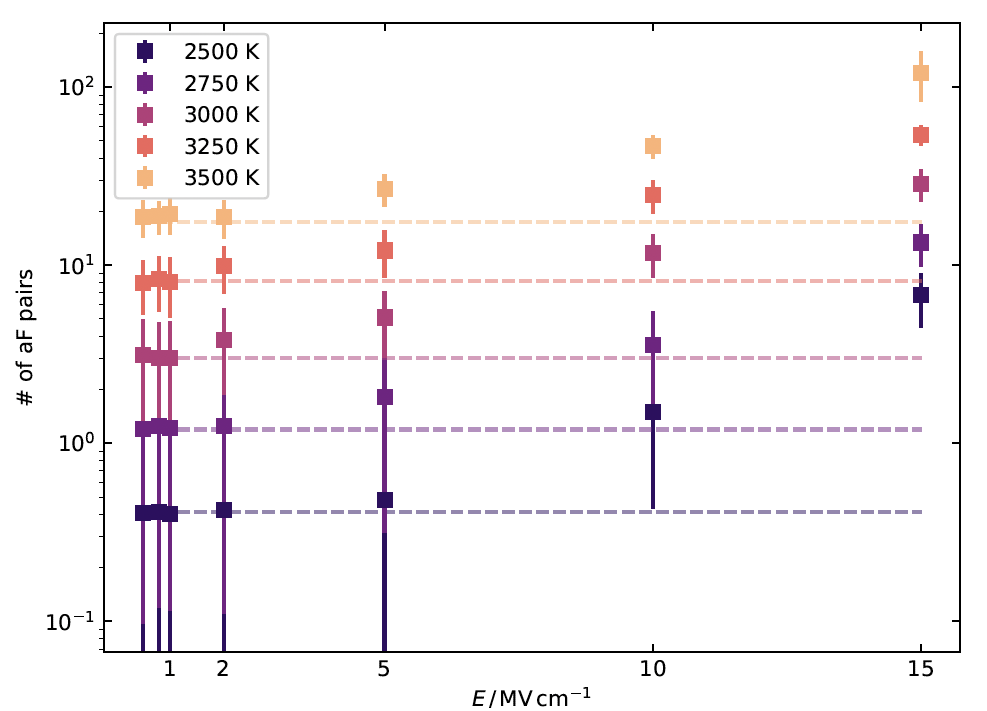 Figure 1: Number of defect pairs formed by an anti-Frenkel (aF) defect formation reaction as a function of electric field strength E in the temperature range (2500- 3500) K. The defect numbers were determined using Wigner-Seitz analysis and averaged over the simulation time. The standard deviation is shown as an error bar. For comparison, the equilibrium defect number at each temperature is shown as a dashed line.
Figure 1: Number of defect pairs formed by an anti-Frenkel (aF) defect formation reaction as a function of electric field strength E in the temperature range (2500- 3500) K. The defect numbers were determined using Wigner-Seitz analysis and averaged over the simulation time. The standard deviation is shown as an error bar. For comparison, the equilibrium defect number at each temperature is shown as a dashed line.